Left ventricular hypertrophy (LVH) is a crucial attribute of hypertensive coronary heart illness. Renin-angiotensin system (RAS) blockers have been proven to be efficient medication for the reversal of LVH.
Clinical and experimental research have proven that Zi Shen Huo Luo Formula (ZSHLF) can enhance the efficacy of perindopril in the therapy of hypertensive LVH, however its mechanism is unclear.
This examine aimed to research the doable mechanism to enhance the efficacy of perindopril. First, we recognized 23 compounds in ZSHLF by extremely efficiency liquid chromatography/tandem mass spectrometry (UPLC-MS/MS) evaluation, amongst which ferulic acid, caffeic acid, vanillic acid, berberine, rutin, quercetin, kaempferol, stachydrine, and tiliroside have been reported to scale back blood strain and exhibit cardioprotective results.
Second, we handled spontaneously hypertensive rats (SHRs) with perindopril and ZSHLF for 12 steady weeks and discovered that power use of perindopril might enhance the aldosterone (ALD) ranges and trigger aldosterone breakthrough (ABT). ZSHLF mixed with perindopril decreased the ALD ranges, interfered with ABT, decreased blood strain, improved left ventricular diastolic dysfunction, and decreased the collagen quantity fraction; these results had been superior to these of perindopril alone.
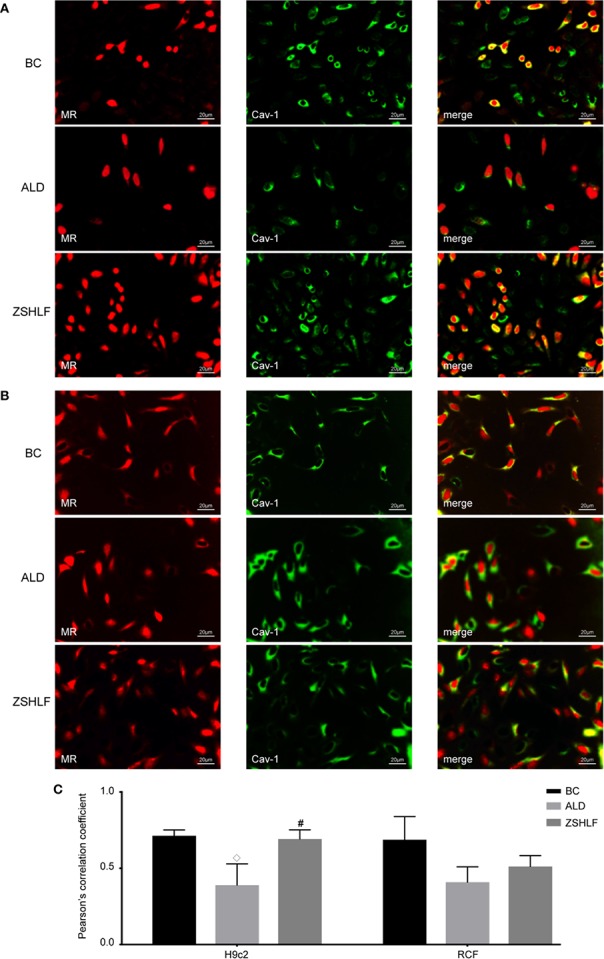
In vitro experiments, ALD-induced cardiomyocytes (H9c2 cells) and cardiac fibroblasts had been handled with ZSHLF-containing serum, which suppressed ALD-induced cardiomyocyte hypertrophy and cardiac fibroblast proliferation, elevated mineralocorticoid receptor (MR) and Cav-1 colocalization and decreased phosphorylated epidermal development issue receptor (pEGFR) and phosphorylated extracellular signal-regulated kinase (pERK) protein expression the cells.
In conclusion, ZSHLF can intervene with ABT and have an effect on the pathological position of ALD by affecting MR and Cav-1 interactions and EGFR/ERK signaling pathway. These results characterize a doable mechanism by which ZSHLF improves the efficacy of angiotensin-converting enzyme inhibitors (ACEIs) in hypertensive LVH therapy. However, the main bioactive parts or metabolites chargeable for the results and the implications of these findings in sufferers want additional verification.

DNA methylation, catalyzed by DNA methyltransferases (DNMTs), is a heritable epigenetic mark, taking part in quite a few physiological processes. DNMT3A is of specific relevance to hematopoietic differentiation, as a result of DNMT3A mutations are strongly associated to hematopoietic malignancies. Additionally, DNMT3A deficiency has been reported to extend the hematopoietic stem cell pool by limiting their differentiation.
Our earlier examine demonstrated that full loss of DNMT3A resulted in anemia, whereas DNMT3A haploinsufficiency prompted an elevated inhabitants of erythrocytes in the content material of oncogenic KRAS. Since erythropoiesis is tightly regulated through the erythropoietin (EPO)-mediated RAS-RAF-MEK-ERK1/2 pathway, the query arises whether or not DNMT3A cooperates with RAS signaling to modulate erythropoiesis.
Human leukemia cell strains had been used, with differentiation capabilities in the direction of megakaryocyte and erythroid lineages. Overexpression of DNMT3A was discovered to boost erythrocytic differentiation of Okay562 cells, whereas DNMT3A knockdown suppressed differentiation. Furthermore, larger DNMT3A expression was detected in late-stage mouse erythroblasts together with the DNMT3A translocation to the nucleus.
Further research demonstrated that each ERK1/2-DNMT3A interplay and serine-255 phosphorylation in DNMT3A led to DNMT3A translocation into the nucleus, and modulated erythrocytic differentiation. Our outcomes not solely discover the important position of DNMT3A in erythropoiesis, but in addition present a brand new perception into ERK1/2-DNMT3A interplay in the hematopoietic system.